truFlex™
Rapid muscle sculpting1
Expand your treatment offerings with truFlex, a muscle-stimulating device that offers personalized treatments based on patient fitness level, shape, and goals in just 15 minutes. Can be paired with truSculpt for full body sculpting treatment.

truFlex in action
Strengthen, tone, and firm abdominal muscles, buttocks, and thighs in as little as 15 minutes.1
truFlex technology
The truFlex Advantage
truFlex features Cutera’s proprietary Multi-Directional Stimulation (MDS), which provides three unique treatment options, covering the largest treatment area in the body sculpting industry.
For device usage and safety guide, click here.
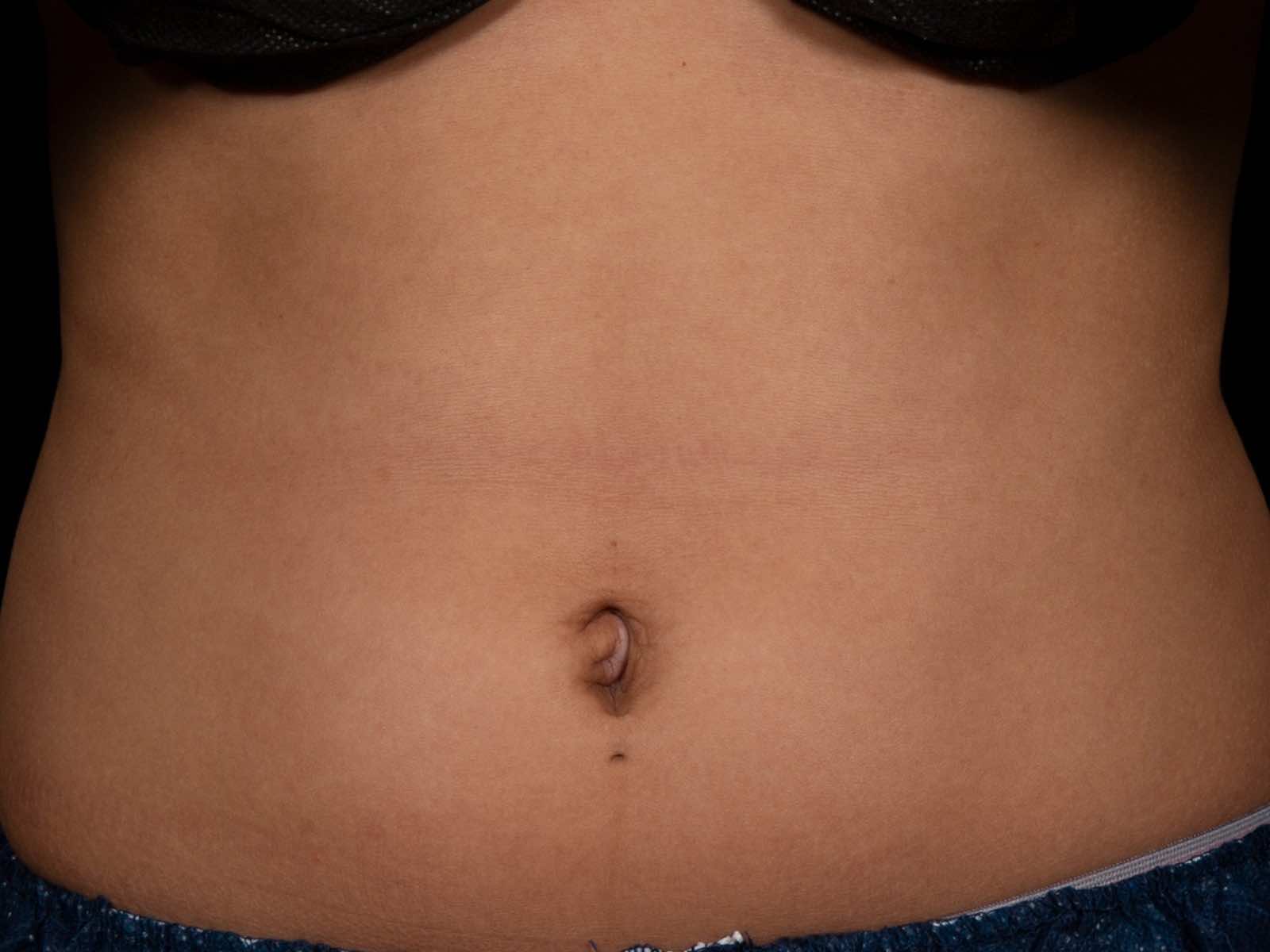
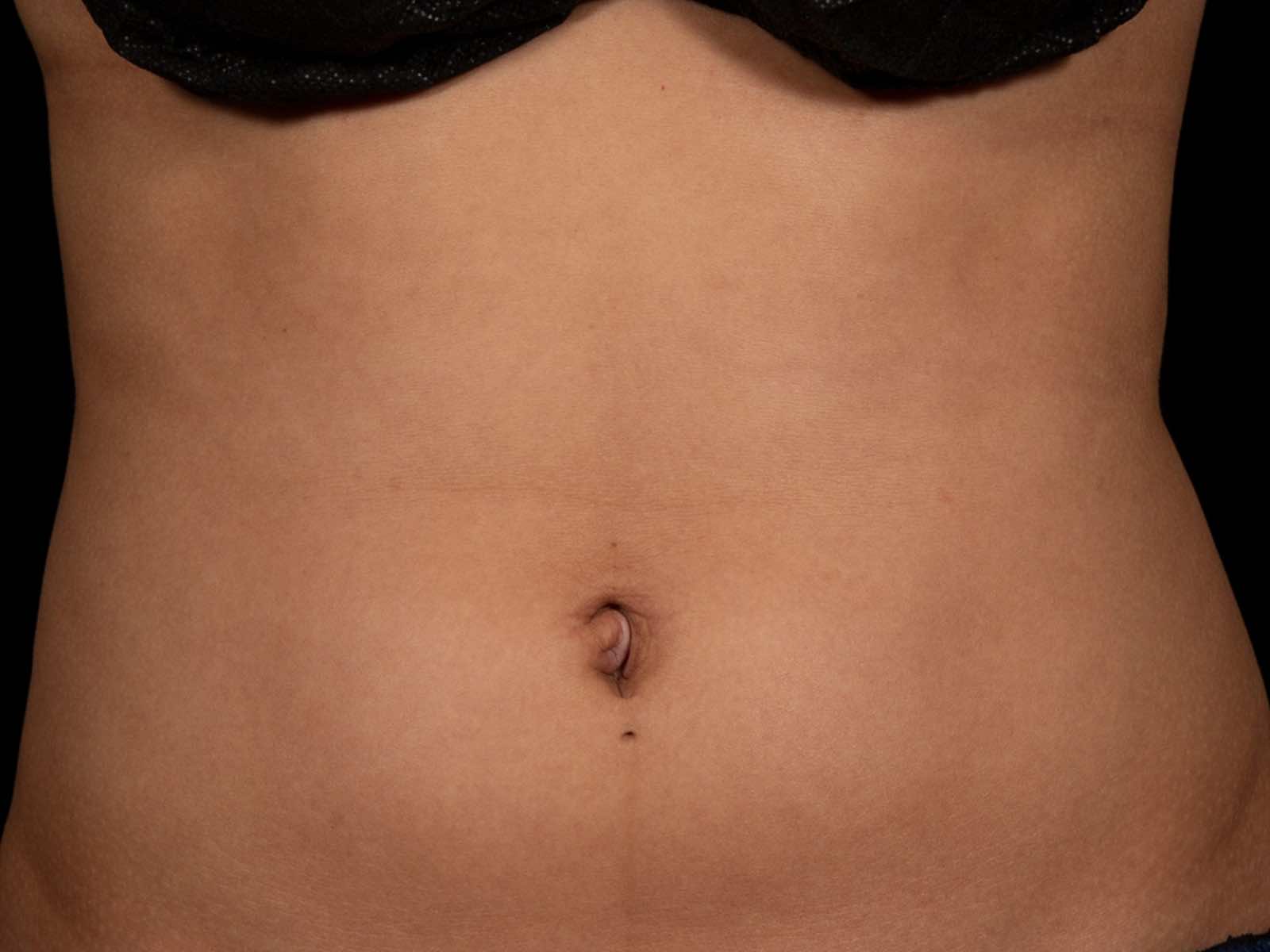
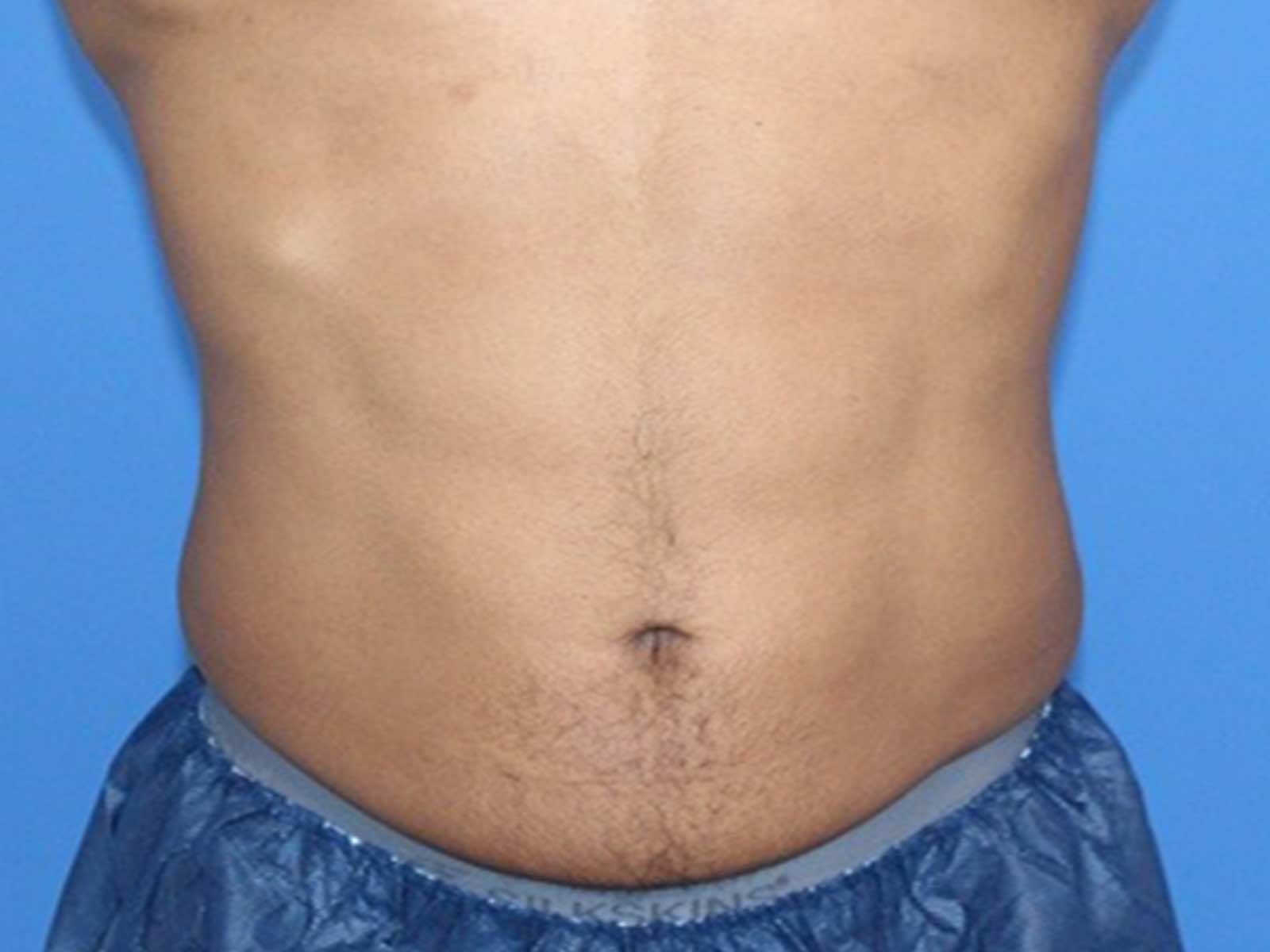
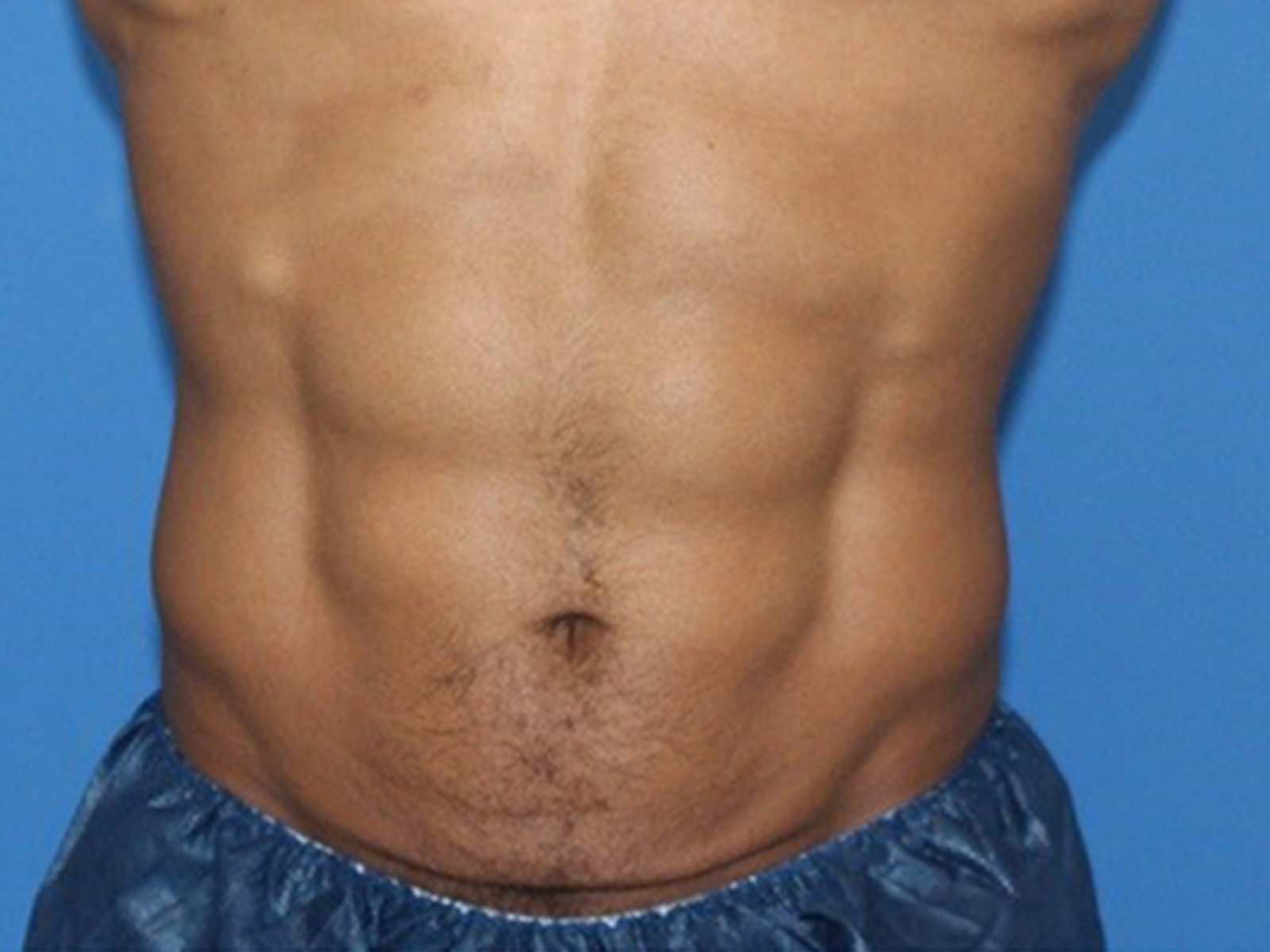
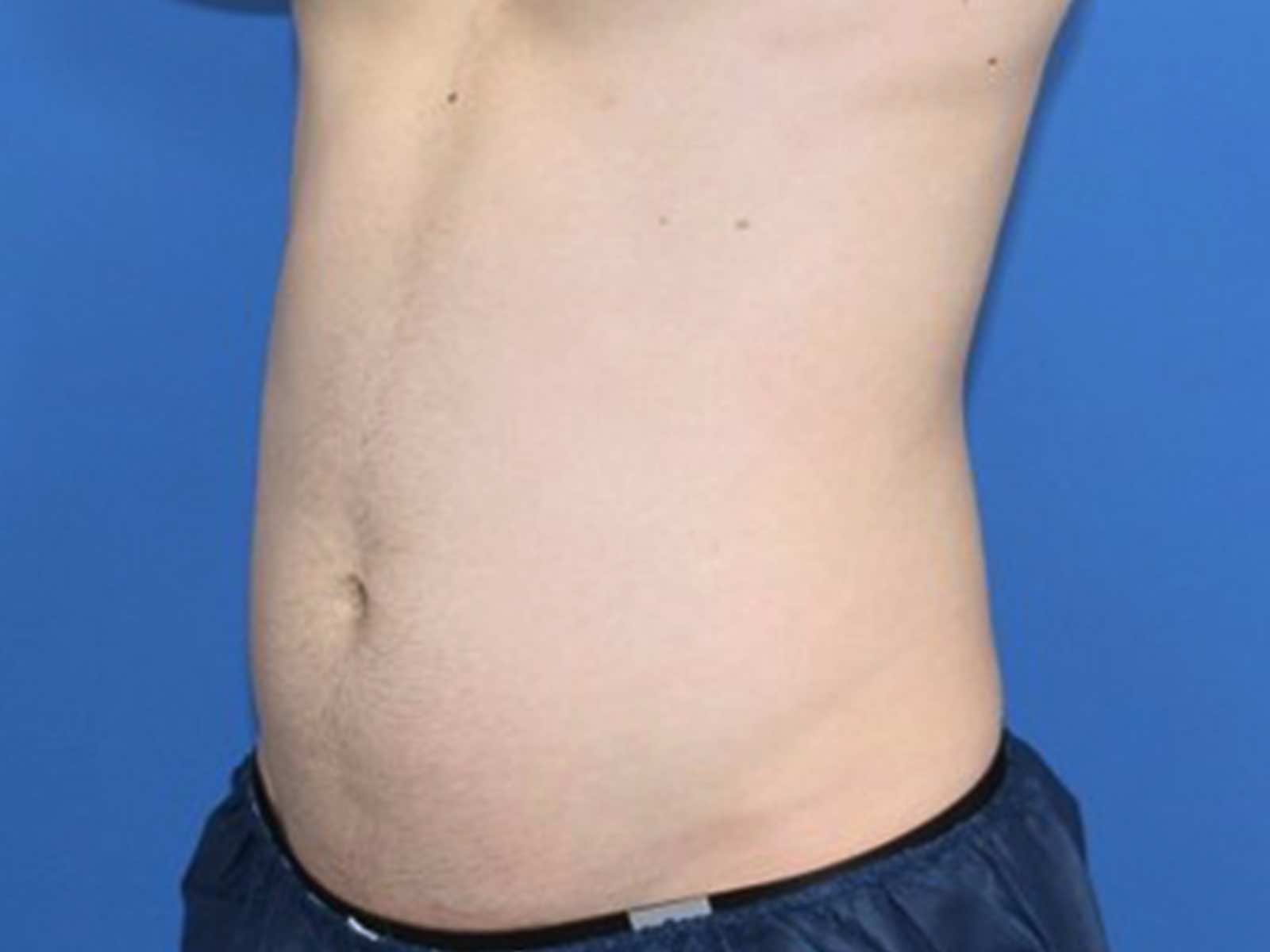
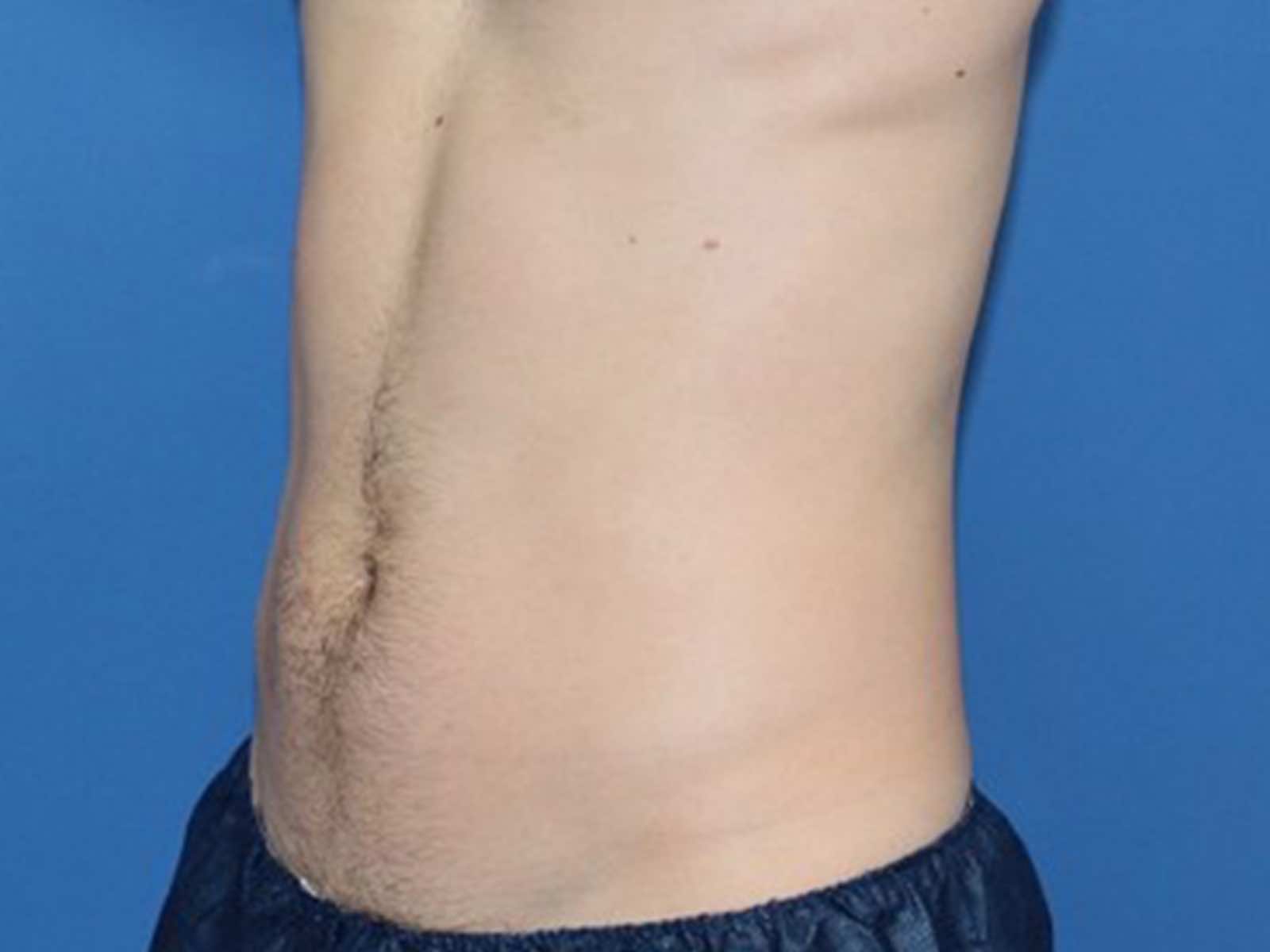
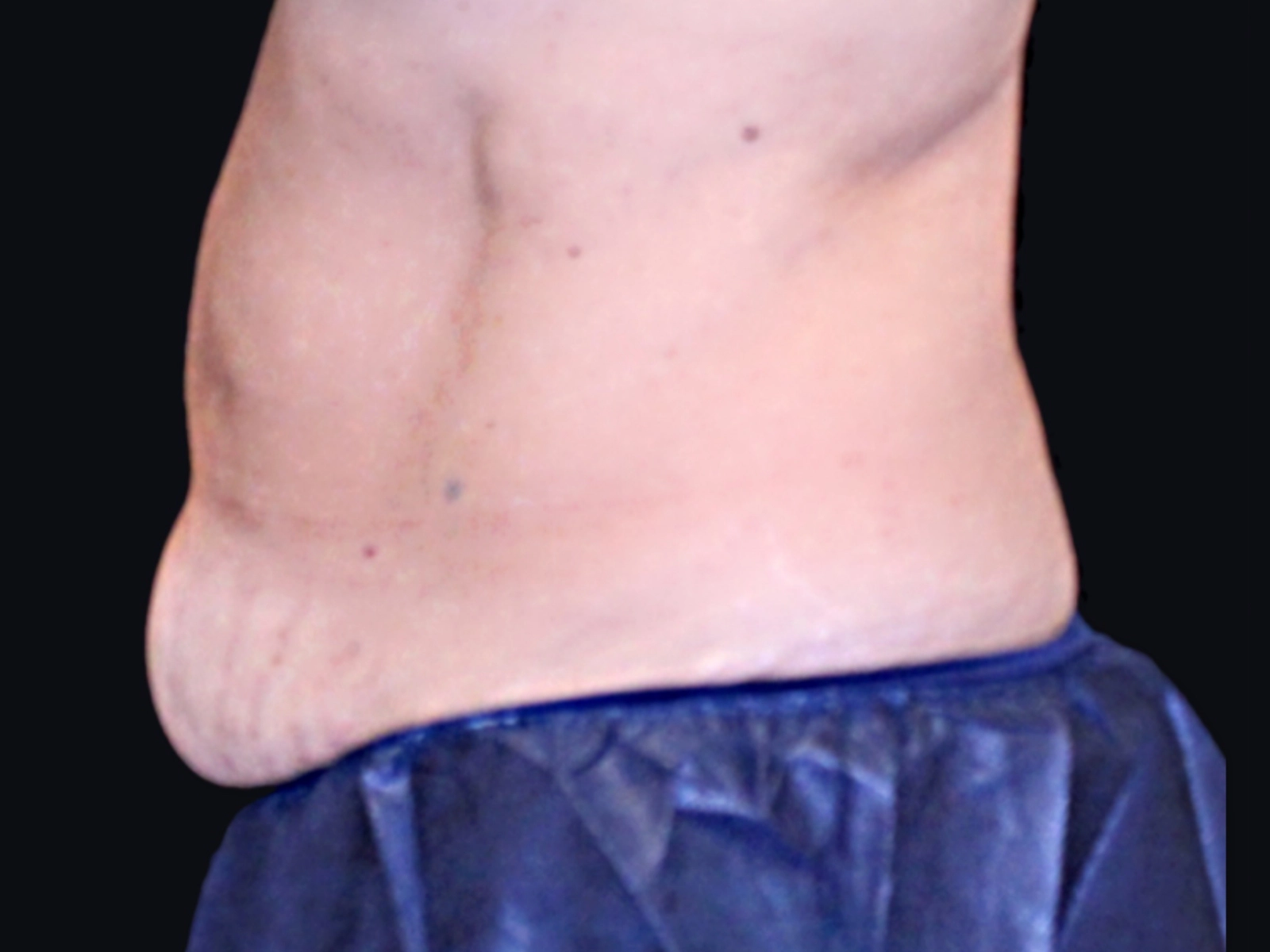
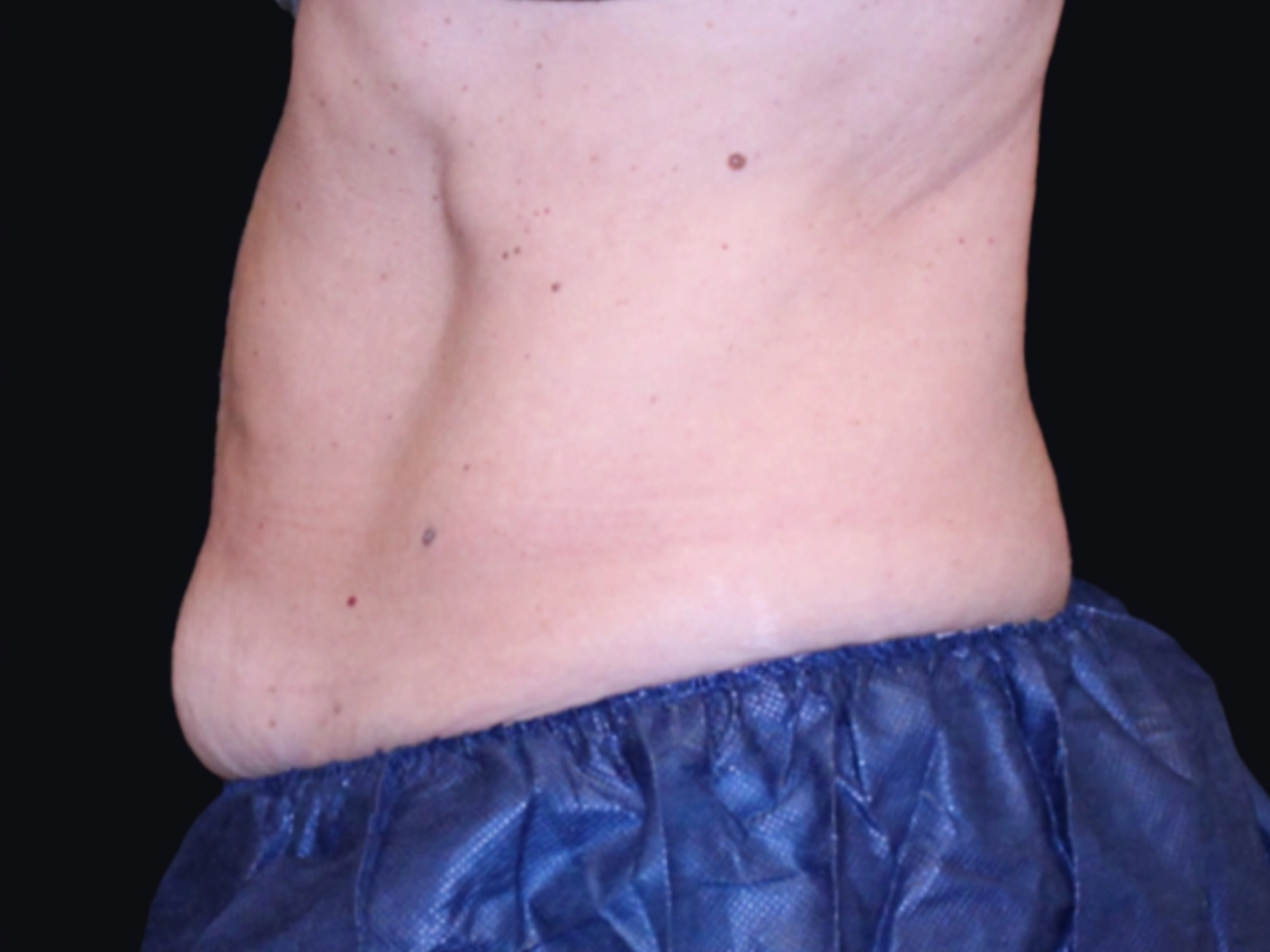
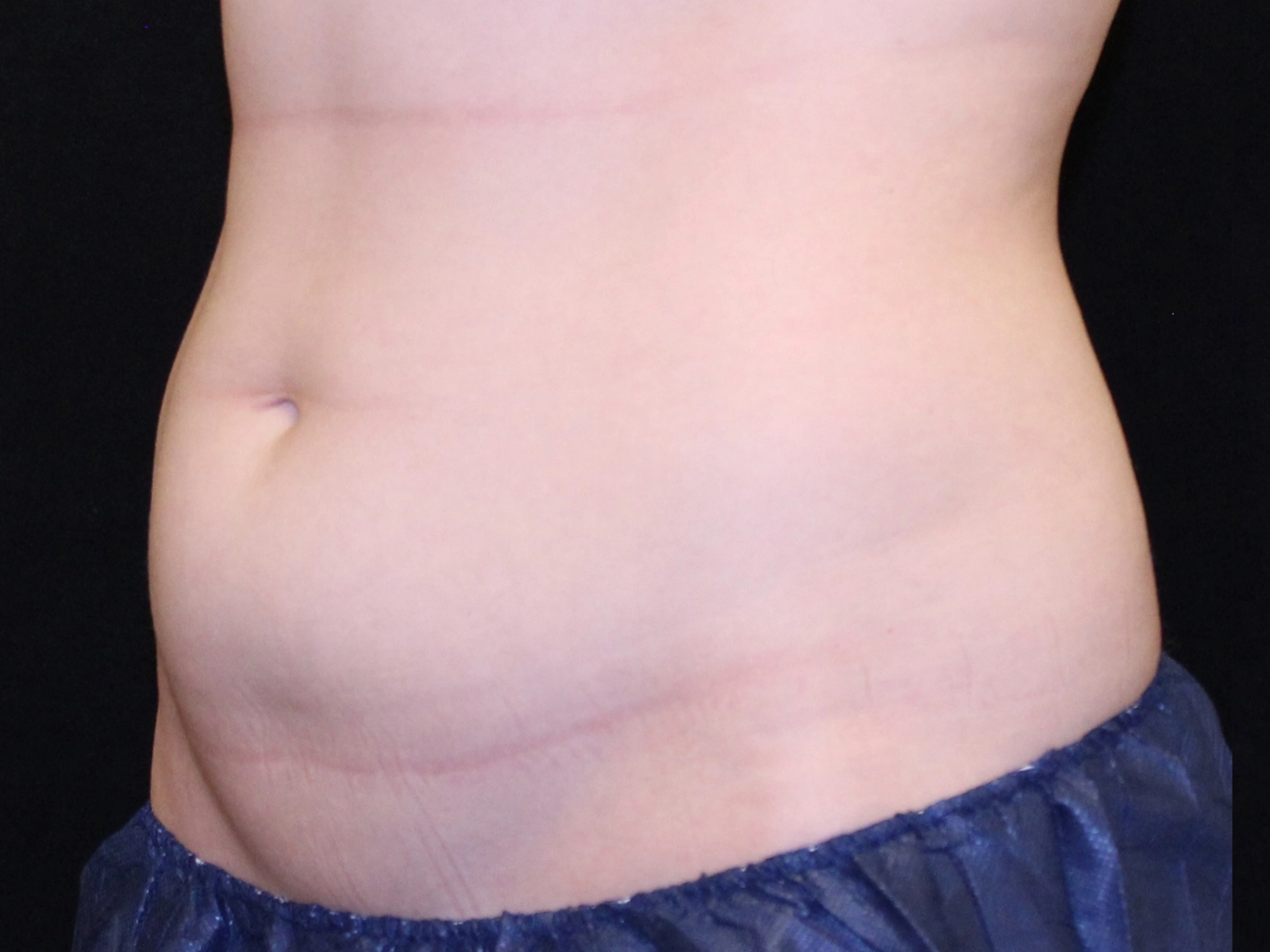
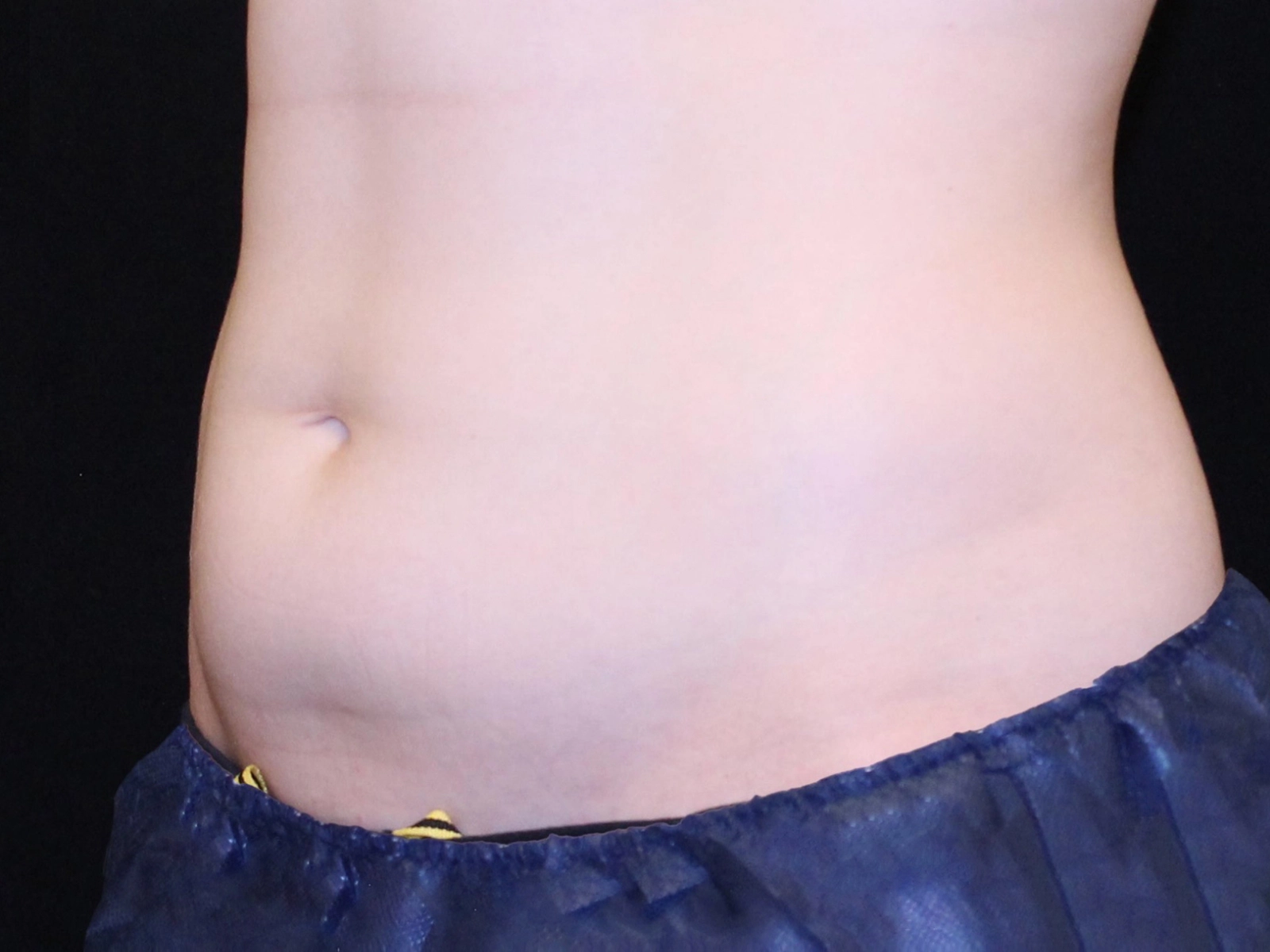
Before and after results
Individual results vary. Images are for illustrative purposes only. A consultation is required to determine suitability.
Hear from our customers
"We can give patients the results they want by targeting individual muscle groups and use handpieces to get rid of fat in very specific areas, no matter where they may be on the body"

"Muscle wasting post-reconstructive joint surgery prolongs rehabilitation. Impressed by Cutera’s research on truFlex’s rapid muscle bulk increase, I experienced significant strength and bulk improvements after four treatments. At SunSculpt on the Sunshine Coast, truFlex has shown excellent results in selected patients, delivering muscle sculpting and toning more rapidly than other treatments."

“I've got a number of clients that had chronic back pain that we've strengthened their core with truFlex and we've seen a significant improvement in their levels of pain and function."

Carousel items
OTHER SOLUTIONS
-
Secret™ PRO
-
Dynamic Facial Resurfacing and Remodeling
Secret DUO
-
xeo®+
-

Skin Revitalization and Resurfacing
Secret RF
-

The First and Original FDA-Cleared Energy Device for the Treatment of Mild to Severe Acne.
AviClear
-

Skin Revitalization and Resurfacing, Pigmented Lesions, Tattoo Removal
enlighten
-

Hair Removal, Pigmented Lesions, Vascular Lesions
excel HR
-
Skin Revitalization and Resurfacing, Pigmented Lesions, Vascular Lesions
excel V
-

Skin Revitalization and Resurfacing, Pigmented Lesions, Vascular Lesions
excel V+
-

Body Sculpting
truSculpt
-
Skin Revitalization and Resurfacing, Pigmented Lesions, Vascular Lesions, Hair Removal
xeo+
1. truSculpt flex Operator Manual Cutera Inc. D0002529, Rev. A, 10/19.