xeo
Skin revitalization and resurfacing, pigmented lesions, vascular lesions, hair removal1,2
A customizable, multi-application laser- and light-based platform designed to treat the widest range of today’s most common nonsurgical aesthetic concerns.


xeo in action
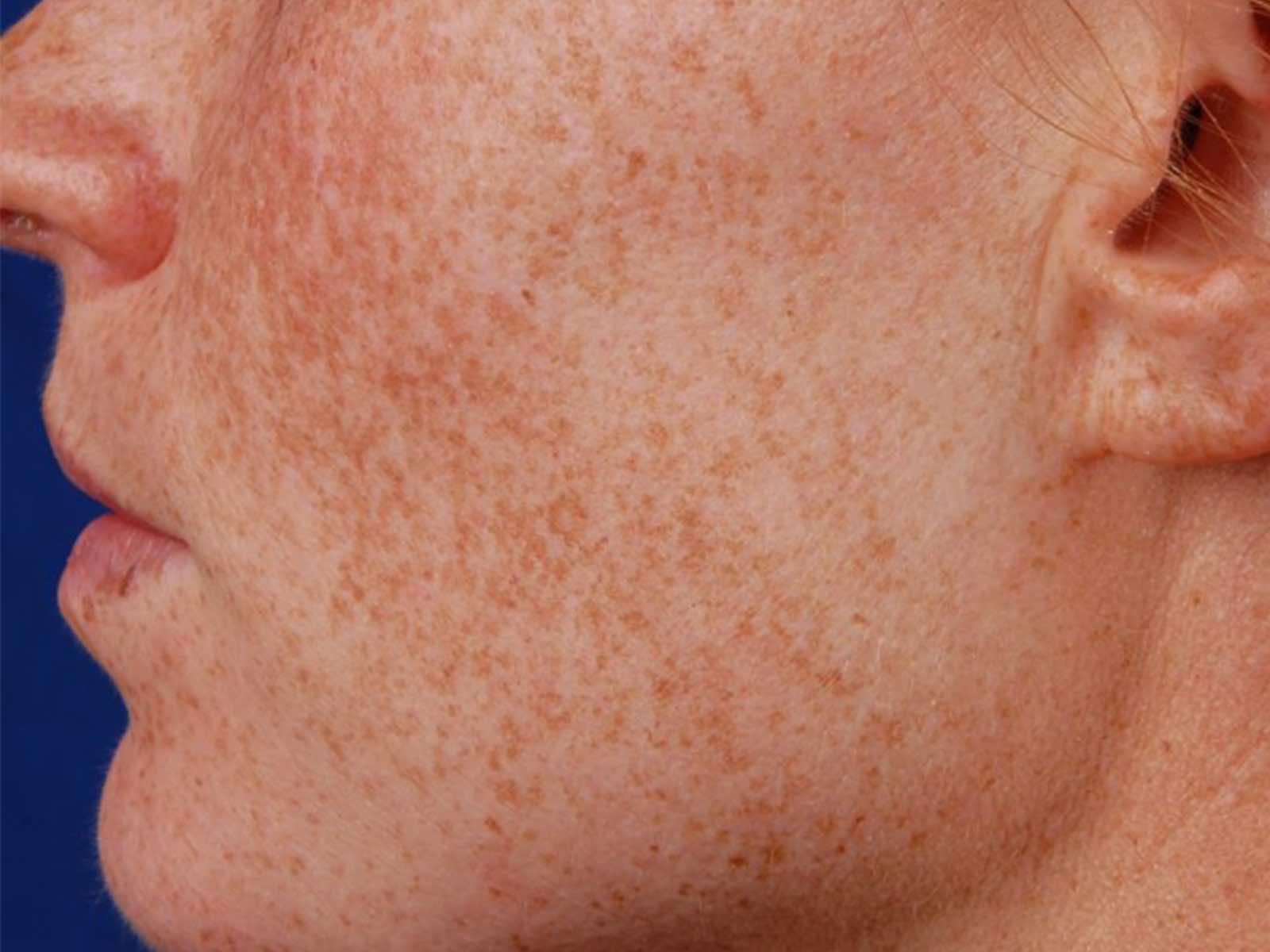
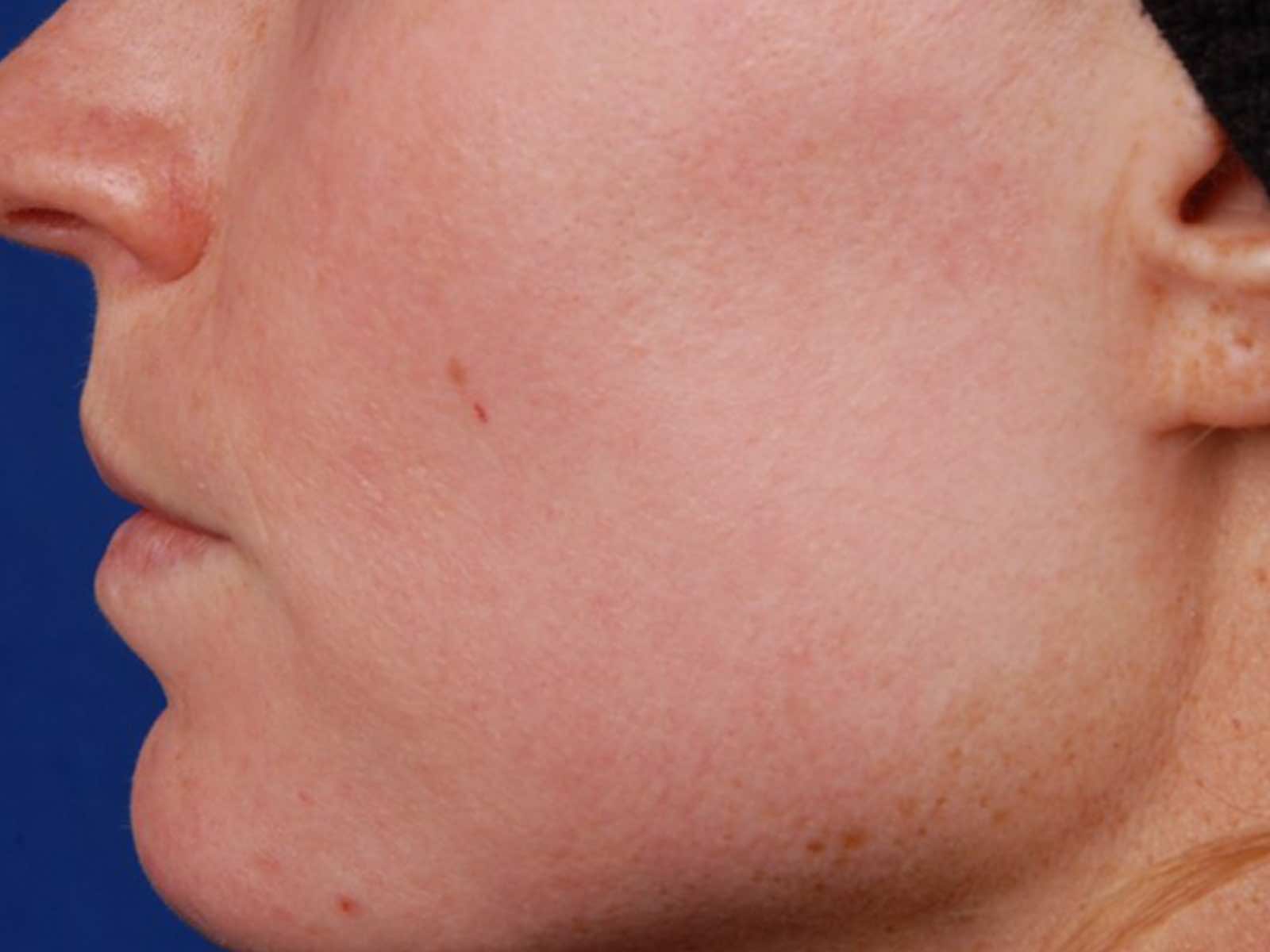
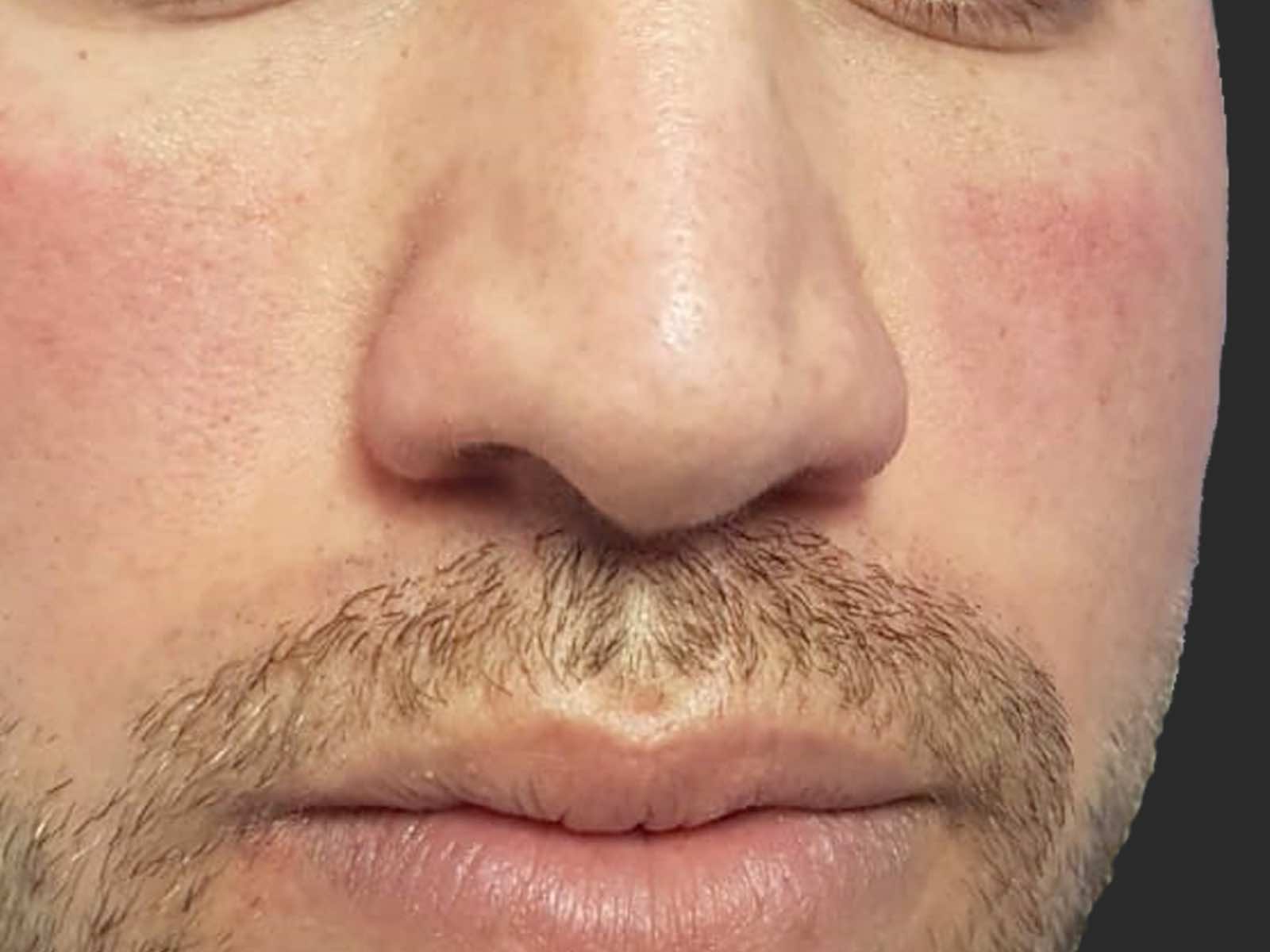
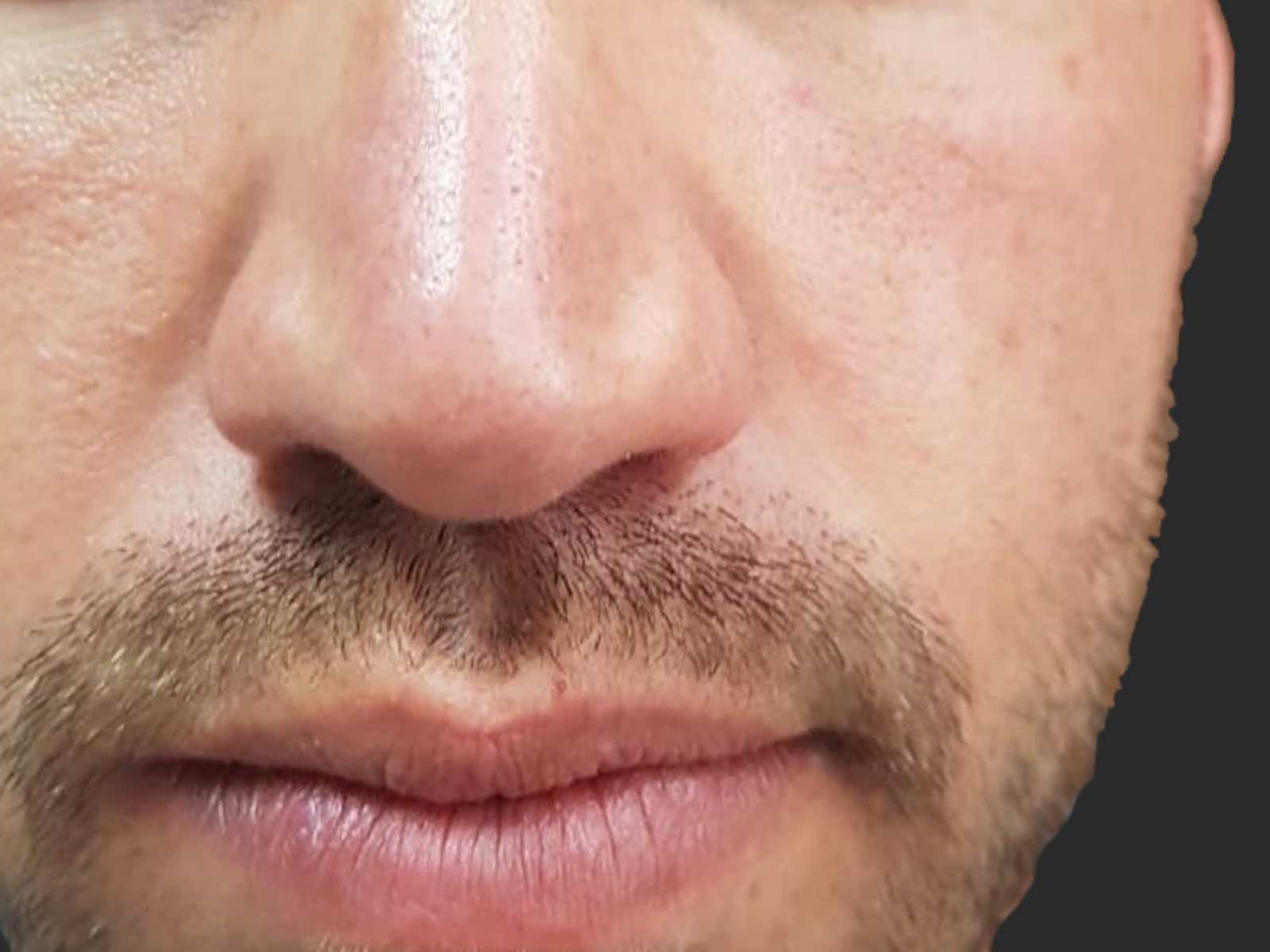
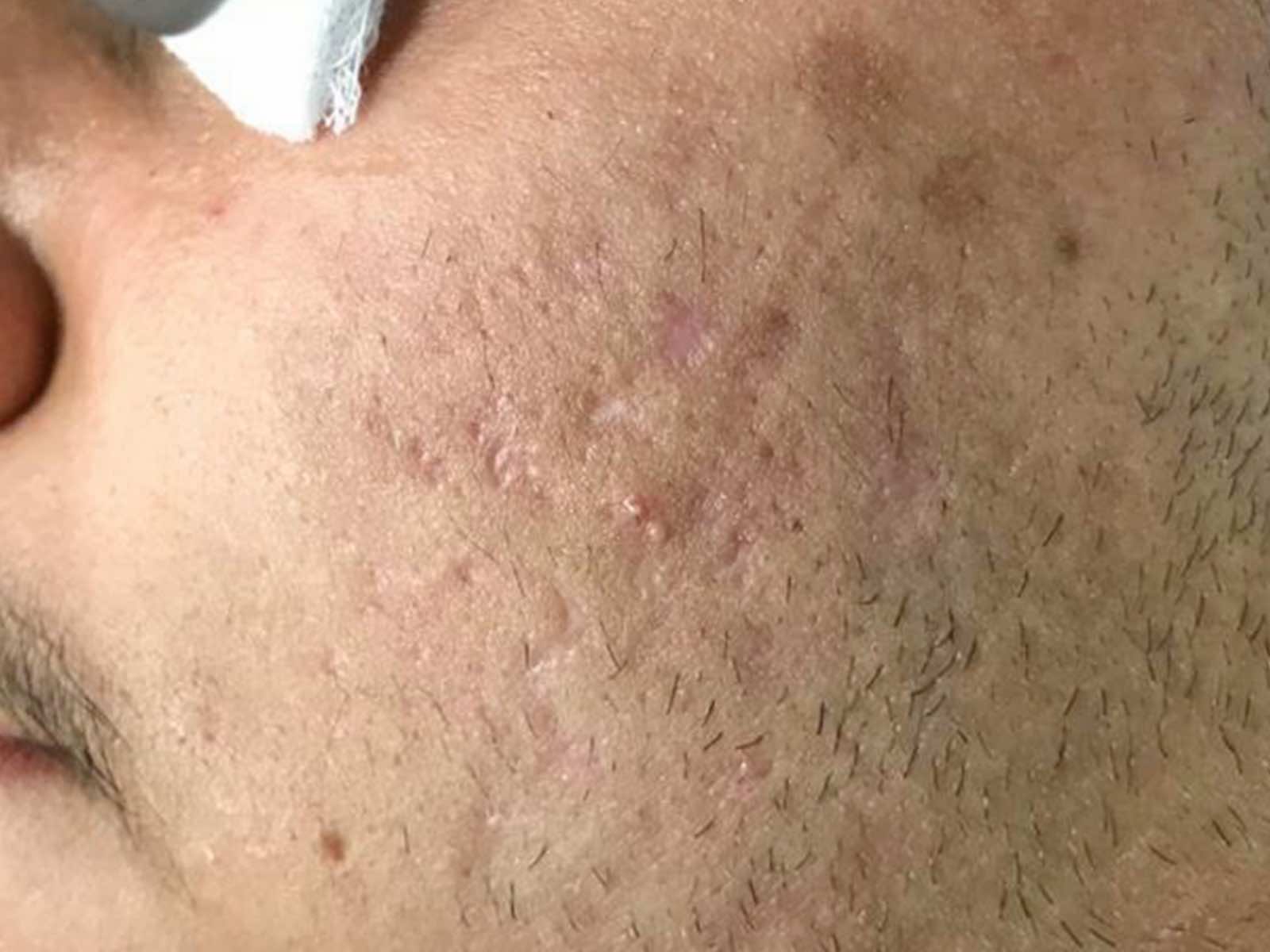
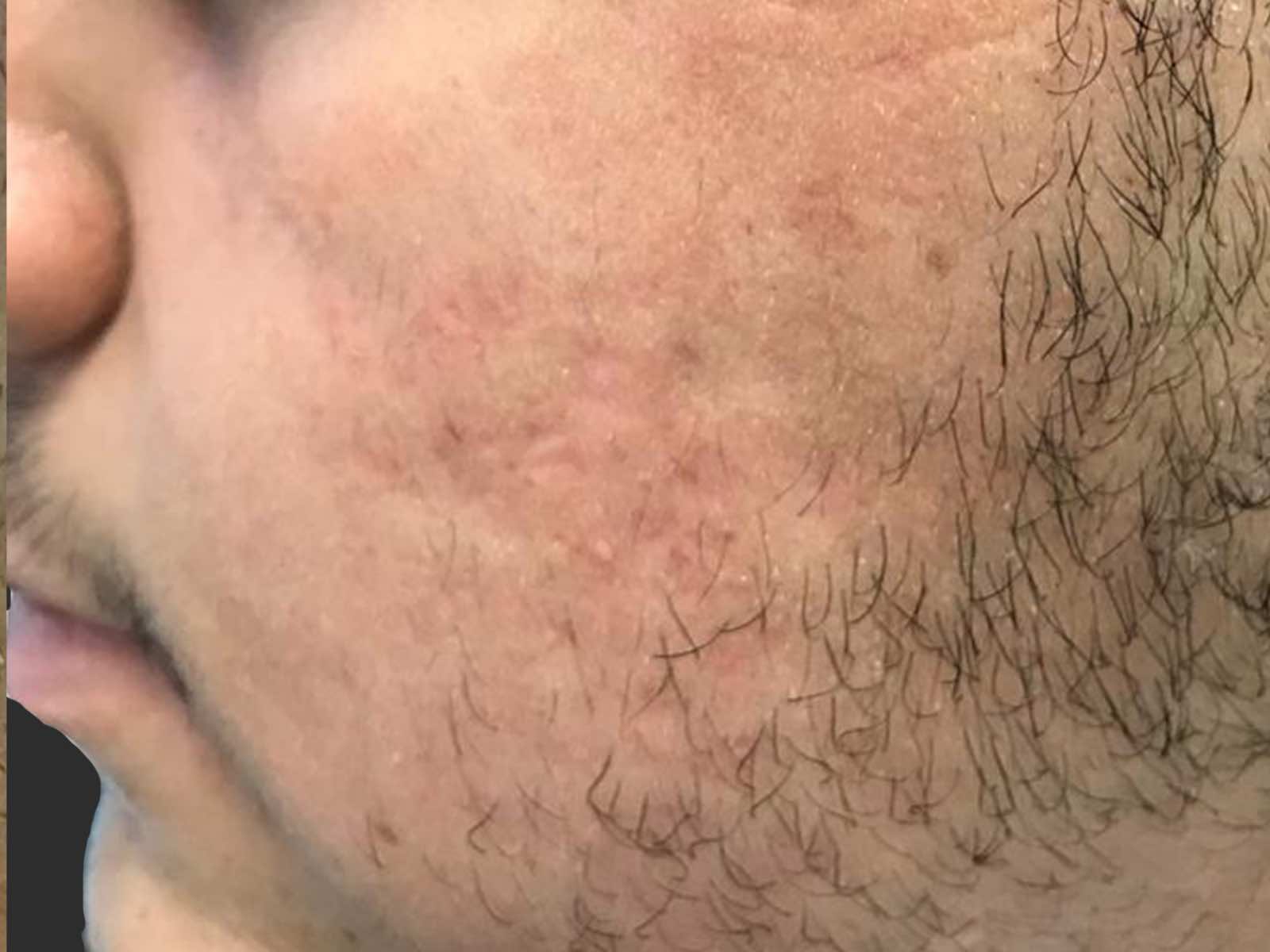
Applications include acne scars, age spots, angiomas, compromised skin, deep dermal heating, freckles, inflammatory active acne, hair removal, lentigines, photodamage, poikiloderma, rosacea, scar reduction, facial veins, leg veins, periorbital veins, venous lakes, warts, and wrinkles.1,2
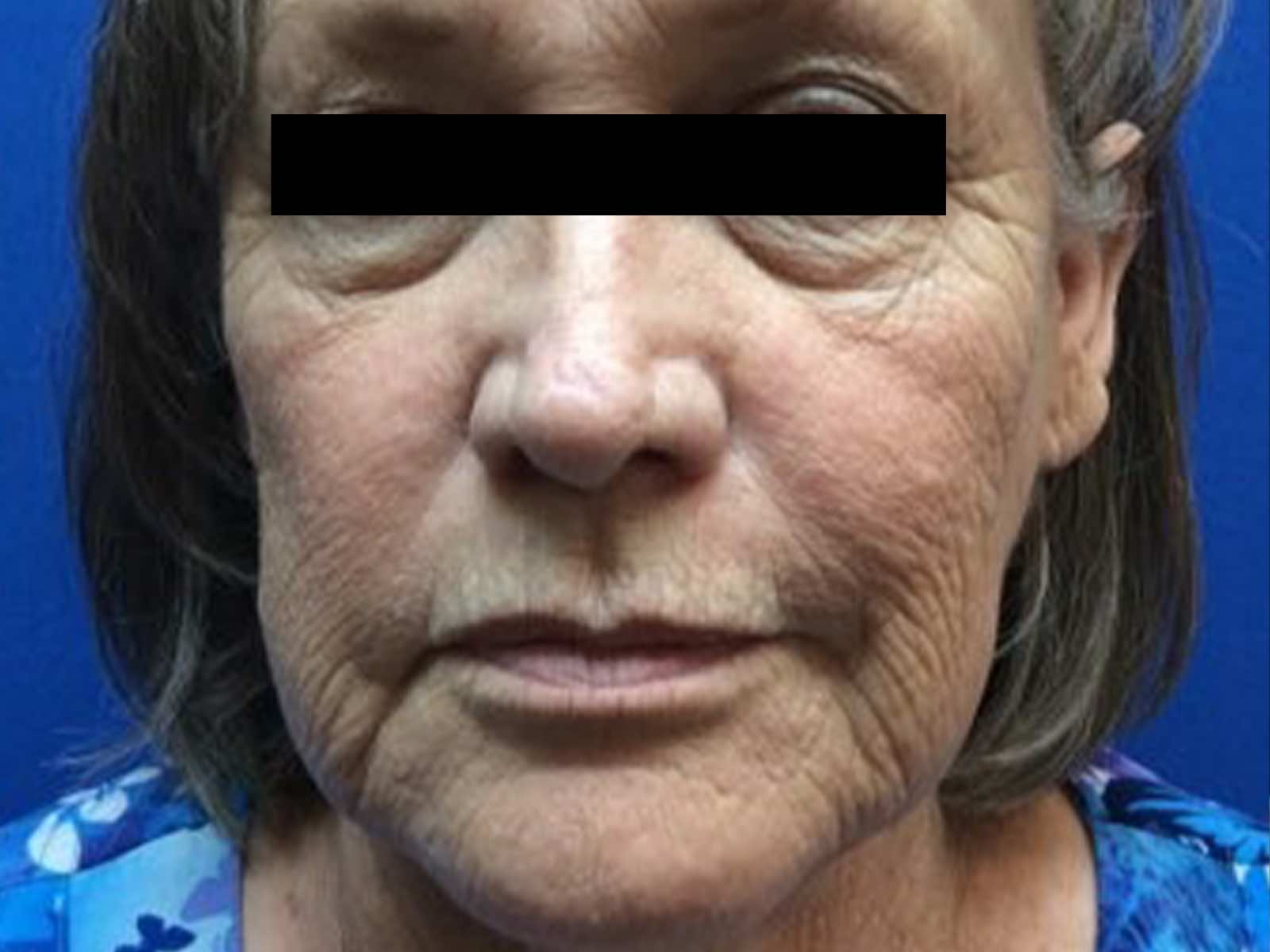
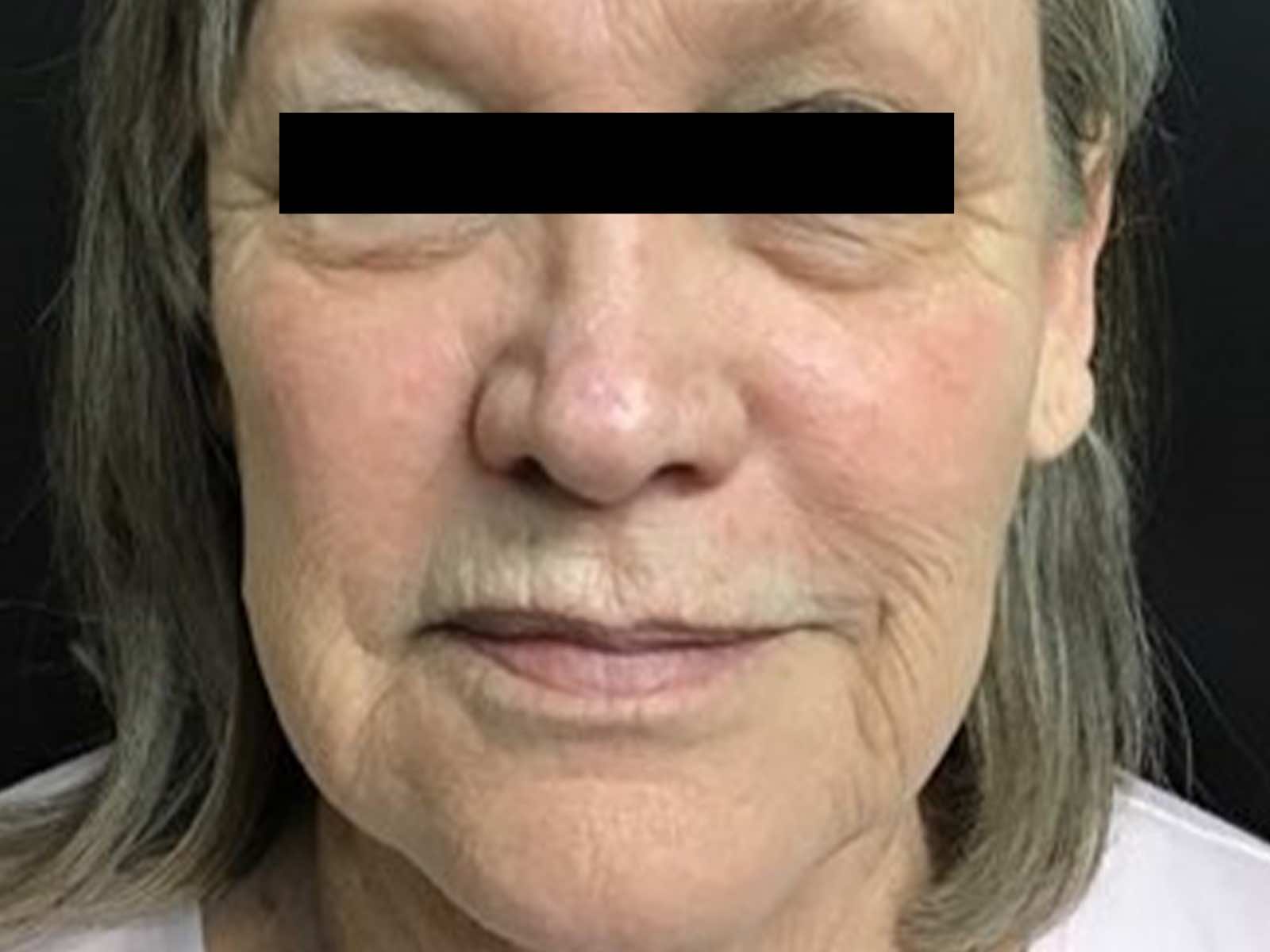
Before and after results
Hear from our customers
“By stimulating collagen, Laser Genesis effectively reduces the appearance of fine lines and wrinkles, improves overall tone and texture on the skin, and helps diminish redness in the treatment area. Beyond an improved complexion, we can treat rosacea and sunspots and lessen the appearance of pores.”
Carousel items
OTHER SOLUTIONS
-

The First and Original FDA-Cleared Energy Device for the Treatment of Mild to Severe Acne.
AviClear
-

Skin Revitalization and Resurfacing, Pigmented Lesions, Tattoo Removal
enlighten
-

Hair Removal, Pigmented Lesions, Vascular Lesions
excel HR
-
Skin Revitalization and Resurfacing, Pigmented Lesions, Vascular Lesions
excel V
-

Skin Revitalization and Resurfacing, Pigmented Lesions, Vascular Lesions
excel V+
-
Dynamic Facial Resurfacing and Remodeling
Secret DUO
-
Skin Revitalization and Resurfacing, Pigmented Lesions, Vascular Lesions, Hair Removal
xeo+
-

Skin Revitalization and Resurfacing
Secret PRO
-

Skin Revitalization and Resurfacing
Secret RF
-

Rapid Muscle Sculpting
truFlex
-

Body Sculpting
truSculpt
Indications for Use: The xeo 1064 nm is indicated for the coagulation and hemostasis of benign vascular lesions, such as, but not limited to, port wine stains, hemangiomas, warts, telangiectasias, rosacea, venous lake, leg veins, spider veins, and poikiloderma of Civatte; and treatment of benign cutaneous lesions, such as, but not limited to, warts, scars, striae, and psoriasis; xeo is also intended for the treatment of benign pigmented lesions, such as, but not limited to, lentigos (age spots), solar lentigos (sun spots), cafe au lait macules, seborrheic keratoses, nevi, chloasma, verrucae, skin tags, keratoses, tattoos (significant reduction in the intensity of black and/or blue/black tattoos), and plaques; additionally, xeo is indicated for pigmented lesions to reduce lesion size, for patients with lesion that would potentially benefit from aggressive treatment, and for patients with lesions that have not responded to other laser treatments; xeo is also indicated for the treatment of wrinkles such as, but not limited to, periocular and perioral wrinkles. xeo is also indicated for temporary and permanent hair reduction . Permanent hair reduction is defined as long-term, stable reduction in hair counts observed at 6, 9, and 12 months after the end of a treatment regime. xeo is also indicated for the treatment for pseudofolliculitis barbae (PFB). xeo is also indicated for the reduction of red pigmentation in hypertrophic and keloid scars where vascularity is an integral part of the scar. xeo is also indicated for treatment of mild to moderate inflammatory acne vulgaris. xeo is indicated for use on all skin types (Fitzpatrick I-VI), including tanned skin. The intended use of the integral cooling system in the xeo laser handpiece is to provide cooling of the skin prior to laser treatment; for the reduction of pain during laser treatment; to allow for the use of higher fluences for laser treatments, such as hair removal and vascular lesions; and to reduce the potential side effects of laser treatments. xeo is indicated for the incision/excision and cutting, ablation, coagulation/hemostasis of soft tissue in me pertormance ot surgical appl1cat1ons in endoscopy/laparoscopy, gastroenterology, general surgery, head and neck/otorhinolaryngology (ENT), neurosurgery, oculoplastics, orthopedics, plastic surgery, pulmonary/thoracic surgery, gynecology (e.g., menorrhagia), and urology. Optional Pulsed Light (IPL) Handpiece Indications: treatment of tattoos, treatment of benign pigmented lesions including dyschromia, hyperpigmentation, melasma, ephelides (freckles), treatment of cutaneous lesions including warts, scars and striae, treatment of benign cutaneous vascular lesions, including port wine stains, hemangiomas, facial, truncal and leg telangiectasias, roasacea, erythema of rosacea, angiomas and spider angiomas, poikiloderma of Civatte, leg veins and venous malformations, treatment of psoriasis, vitiligo, atopic dermatitis (eczema), seborrheic dermatitis, removal of unwanted hair from all skin types, and to effect stable long-term, or permanent, hair reduction, treatment of mild to moderate inflammatory acne vulgaris.
Important Safety Information: The following treatment-related expected transient side effects and possible adverse events may occur during or following treatment with xeo: Erythema; Edema; Purpura; Hyperpigmentation; Hypopigmentation; Burns; Erosion; Epidermal crusting; Blistering; Scarring; Deep tissue injury; Prolonged wound healing; Temporary or permanent gray hair; Stimulation of terminal hair growth; Undesired hair loss in hair-bearing areas; Red rash/bumps; Hemosiderin staining; textural changes; Cutaneous indentations; Sun exposure, tanning beds, and artificial tanning may increase the risk of side effects and adverse events. Do not use xeo if you are pregnant or are undergoing treatment for skin cancer. Additional contraindications can be discussed during a treatment consultation.
Contraindications: The xeo series of systems is contraindicated for: pregnant patients; patients undergoing treatment for skin cancer.
Warnings: Do not treat over dysplastic nevi or questionable pigmented lesions. Do not treat over open wounds. Do not treat over or close to tattoos or permanent make-up. Do not treat over or close to metal or electronic implants. Hair removal by lasers or intense pulse light sources can cause increased hair growth in some individuals. Based upon currently available data, the highest risk groups for this response are females of Mediterranean, Middle Eastern, and South Asian heritage treated on the face and neck.
Precautions: Use caution when treating patients with any of the following: History of coagulopathies; History of keloids or hypertrophic scarring; History of herpes – pre-treatment with an antiviral may be indicated History of vitiligo – may cause de-pigmentation; Diabetes – may impede wound healing Metal or electronic implants; Current or recent use of the following medications: Accutane – do not treat if taken in the past 6 months; Gold Therapy – may cause blue-gray discoloration; Anticoagulants – may increase risk of purpura or bruising; Photosensitizing medications/agents. In addition, observe the following precautions: Use caution when treating over hair bearing areas. Laser energy may affect hair growth. Place moist gauze between the lips and teeth if treating near the mouth. Laser energy may affect teeth. Treat at least at least 15 cm (6 in) from pacemakers/defibrillators. Reaction to metal or electronic implants is unknown. Refer to the Operator Manual to view the laser safety labels of the product.”